
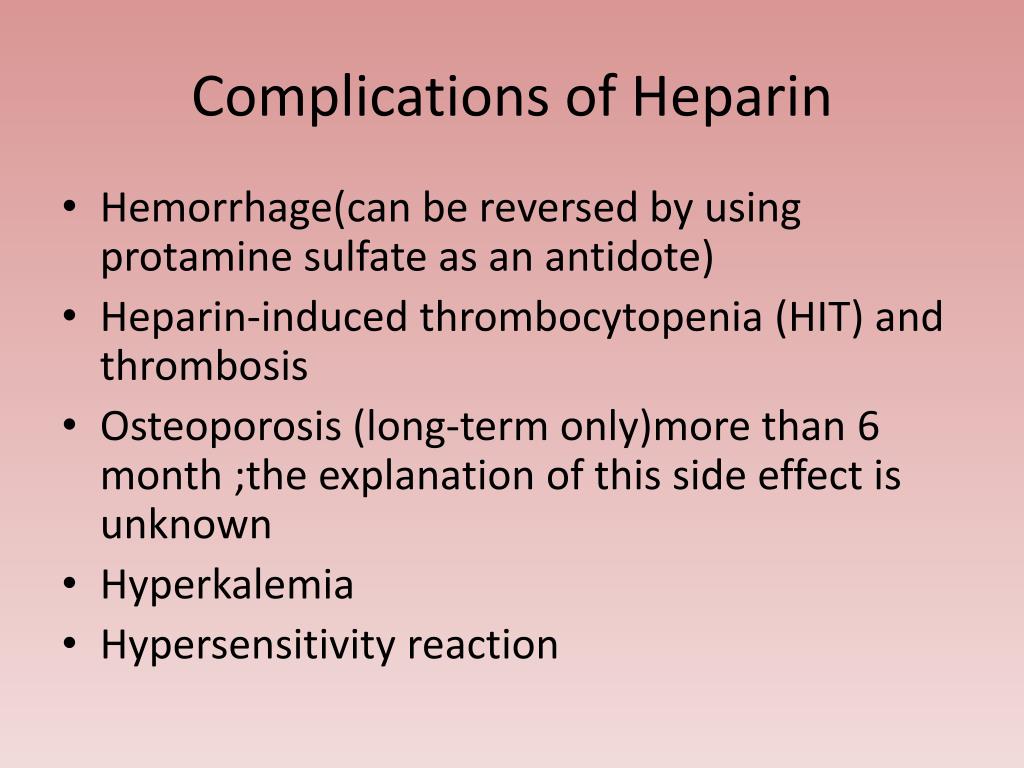
4 After reversal, dabigatran activity was nearly undetectable in 99.4% of the patients in RE-VERSE AD, whereas more than a quarter of the rivaroxaban-treated patients in ANNEXA-4 maintained concentrations above the threshold, suggesting a limited binding capacity of andexanet. The aim of reversal is to reduce anticoagulant activity below a safety hemostatic threshold, usually defined by international normalized ratio <1.3–1.5 for vitamin K antagonists and 50 ng/mL for DOACs. First the immediate biological efficacy is questionable. These encouraging results come with caveats. This single-arm study confirmed that a bolus of andexanet followed by a 2-hour infusion markedly reduced anti-FXa activity (92% reduction) in patients with acute major bleeding receiving FXa inhibitors, mainly apixaban and rivaroxaban.
ANTIDOTE TO HEPARIN FULL
The European Medicine Agency waited for the full cohort results in 2019 to grant a conditional marketing authorization. The Food and Drug Administration granted accelerated approval for patients treated with apixaban or rivaroxaban, when reversal is needed because of life-threatening or uncontrolled bleeding, beginning in May 2018 after the intermediate analysis of the ANNEXA-4 study (The Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors study). It has no membrane-binding Gla-domain, so it is unable to assemble into the prothrombinase complex. It has no active site and is therefore catalytically inactive. 3Īndexanet alfa (Andexxa, Portola) is a genetically modified FXa that acts as a decoy to bind FXa inhibitors, including apixaban, rivaroxaban, and edoxaban, but also low-molecular-weight heparins. Indeed, the single-arm RE-VERSE AD study (Reversal Effects of Idarucizumab on Active Dabigatran) demonstrated that intravenous infusion of 5 g idarucizumab provided immediate, effective (100% median maximum percentage reversal), and sustained reversal of anticoagulant activity in 503 dabigatran-treated patients who had uncontrolled bleeding or faced emergency surgery. The Food and Drug Administration approved idarucizumab for dabigatran reversal in life-threatening or uncontrolled bleeding, or emergency procedures. Idarucizumab (Praxbind, Boehringer Ingelheim) is a humanized monoclonal antibody fragment with structural analogies with thrombin that specifically binds dabigatran with high affinity, forming complexes cleared by the kidneys. Ideally, the perfect antidote should induce immediate, complete, and sustained reversal of the anticoagulant activity correlated with clinical improvement, with no side effects, especially thromboembolic, should be user friendly, and should be available at an acceptable price.

2 This is based on the assumption that by reversing the anticoagulant effects, the antidote restores hemostasis, stops hemorrhage, and therefore reduces mortality. Because rapid vitamin K antagonist reversal reduces mortality of patients facing major bleeding and minimizes hematoma expansion after intracerebral hemorrhage, immediate reversal of DOAC should similarly improve outcomes. However, anticoagulant reversal is unquestionably required in specific situations including urgent invasive procedures, overdose, or life-threatening traumatic or spontaneous bleeding. The short half-life of DOACs compared with warfarin obviates the use of an antidote in most cases. Major bleeding occurs annually in 1% to 3% of DOAC-treated patients and results in high mortality. 1 The results of this study question whether these antidotes fulfill an unmet need and improve DOAC-treated patient outcomes. Recently, after the publication of the ANNEXA-4 study, andexanet alfa, the antidote for factor Xa (FXa) inhibitors, was approved. Idarucizumab was marketed in 2018 for the reversal of the thrombin inhibitor dabigatran.

Nevertheless, the fear of bleeding owing to the lack of a specific antidote has been a major concern. Customer Service and Ordering Informationĭirect oral anticoagulants (DOAC) are recommended as the preferred option for the treatment and prevention of thromboembolic events because of a favorable benefit–risk profile when compared with vitamin K antagonists.Stroke: Vascular and Interventional Neurology.
Journal of the American Heart Association (JAHA).Circ: Cardiovascular Quality & Outcomes.Arteriosclerosis, Thrombosis, and Vascular Biology (ATVB).


 0 kommentar(er)
0 kommentar(er)
